2025年5月,国家血液系统疾病临床医学研究中心、苏州大学附属第一医院、苏州大学造血干细胞移植研究所的吴德沛教授和徐杨教授团队在GUT杂志(IF=23.1)在线发表了题为“Commensal Bacteroides T6SS alleviate GI-aGVHD via mediating gut microbiota composition and bile acids metabolism”的文章,首次系统性揭示了肠道共生菌利用六型蛋白分泌系统调节肠道稳态,减轻造血干细胞移植后急性移植物抗宿主病的作用机制。


造血干细胞移植(allo-HSCT)是治疗多种血液系统恶性肿瘤的核心手段。然而,移植后供体免疫细胞将宿主正常组织识别为异物,引发急性移植物抗宿主病(aGVHD),严重威胁患者生命安全并降低生存质量。胃肠道作为aGVHD的主要靶器官之一,其肠道菌群的组成与动态变化对aGVHD的发生发展具有关键影响。已有研究证实,通过粪便微生物群移植(FMT)重建肠道微生态稳态,可显著缓解aGVHD进展。近年研究进一步聚焦肠道有益菌及其代谢产物在aGVHD防治中的作用,但其与宿主免疫反应的复杂互作机制仍未完全阐明,亟待深入探索菌群-宿主的相互作用机制。
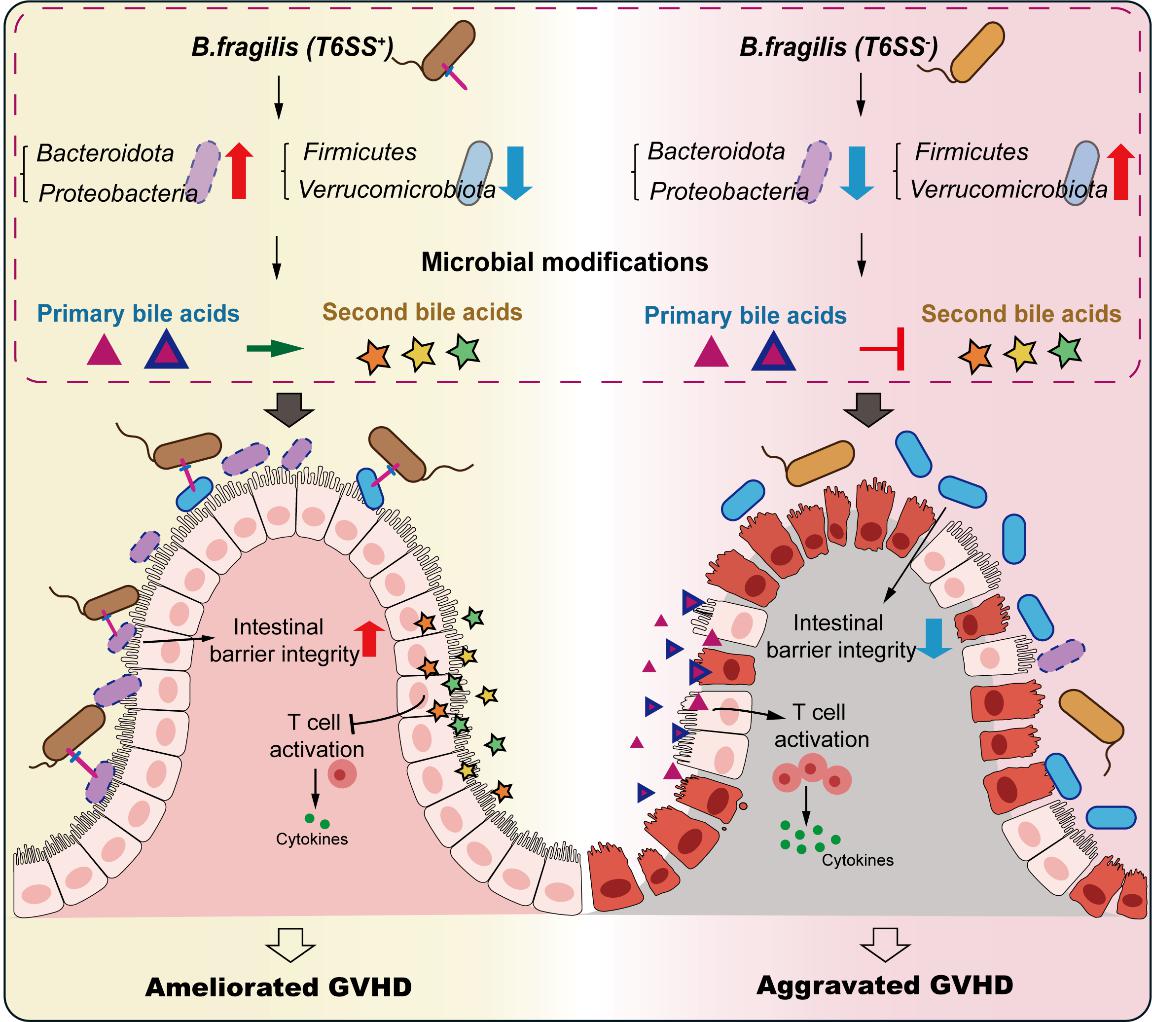
本研究对71例接受allo-HSCT的患者进行了肠道微生物群、免疫指标及肠道代谢特征的系统分析。临床数据表明,拟杆菌属(Bacteroides)丰度升高与患者总生存时间延长、aGVHD累计发生率降低及无GVHD-无复发生存期(GRFS)延长显著相关。值得注意的是,拟杆菌属中广泛存在的六型分泌系统(T6SS)保守基因在非aGVHD发病组中高表达,且其表达水平与患者预后呈正相关。为验证脆弱拟杆菌(B. fragilis)T6SS对aGVHD的调控作用,研究构建了小鼠aGVHD模型,并结合16S扩增子(ASV)测序和代谢组学技术,解析T6SS对肠道微生物结构及代谢组的调控机制。结果显示,T6SS介导细菌间拮抗作用重塑肠道菌群结构,显著影响肠道代谢组,尤其是胆汁酸代谢通路,从而减轻肠道炎症反应、保护肠黏膜屏障完整性,并最终缓解小鼠aGVHD症状。
回归临床数据,在整个allo-HSCT过程中,患者粪便样本均表现出总胆汁酸含量升高与胆汁酸盐水解酶(bsh)基因丰度降低的特征,且胃肠道aGVHD(GI-aGVHD)组的这一表型更为显著。进一步的中介分析揭示了潜在的“T6SS–初级胆汁酸代谢–aGVHD”因果通路,表明对初级胆汁酸代谢的调控是T6SS影响aGVHD发生发展的重要中介。
理解维持微生物稳定性的机制对于开发有效的治疗干预措施至关重要。本研究表明T6SS和胆汁酸代谢是维持肠道稳态的关键内在机制,使其有望成为治疗干预aGVHD的潜在靶点,为临床实践中开发更安全、更有效的微生物疗法提供新的理论支持。
该研究得到国家重点研发计划、国家自然科学基金委、国家血液系统疾病临床医学研究中心、江苏省科技厅等多个科研资金的支持。吴德沛教授、徐杨教授、林丹丹副研究员、刘海燕教授为本文共同通讯作者,李鹏飞、雷棋怡、余星皓、沈莹、陈艺尹为本文的共同第一作者。
原文摘要:
Background Gastrointestinal acute graft-versus-host disease (GI-aGVHD) is one of the main complications of patients undergoing allogenic hematopoietic stem cell transplantation (allo-HSCT). A deeper understanding of the mechanisms of sustaining intestinal homeostasis is essential.
Objective Here we investigated microorganisms and microbial metabolites that were crucial for intestinal homeostasis in the context of GI-aGVHD management.
Design We profiled the gut microbiota, immune indices, and gut metabolism of 71 patients undergoing allo-HSCT. Initially, we set up mouse aGVHD model to confirm the effect ofB. fragilis T6SS on aGVHD progression. Subsequently, we applied 16S ASV sequencing and metabolic sequencing to reveal the function ofB. fragilis T6SS on microbial structure intestinal and metabolome. Finally, the mediation package was used to validate our findings in clinical samples.
Results A higher abundance ofBacteroides spp. contributes to reducing the incidence of GI-aGVHD, and the Type VI Secretion System (T6SS) is required forBacteroides spp. protection on aGVHD. T6SS-mediated antagonism regulates the structure and composition of gut microbiota, affecting the entire gut metabolome, particularly the bile acids metabolism, subsequently reducing inflammation response in the intestine and protecting intestinal barrier integrity. Notably, accumulating primary bile acids such as chenodeoxycholic acid exacerbated aGVHD by enhancing the activation of T cells. Mediation analysis further validated that T6SS affects the incidence of GI-aGVHD through its effect on primary bile acid metabolism.
Conclusion T6SS in commensal bacteria could modulate bile acid metabolism, potentially impacting aGVHD outcomes and offering a novel target for therapeutic interventions.
原文链接:https://gut.bmj.com/content/early/2025/05/28/gutjnl-2024-334565?rss=1